Welcome to qPhos
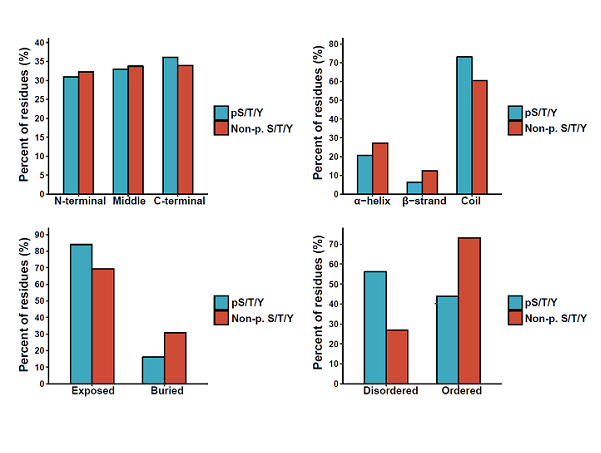
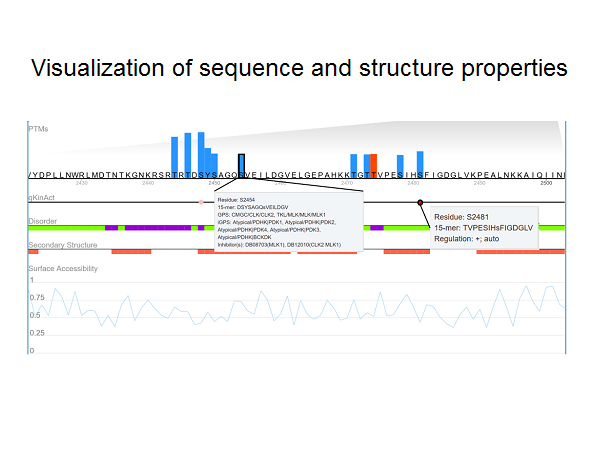
Protein phosphorylation is one of the most important post-translational modifications (PTMs) and regulates a broad spectrum of biological processes. Especially, phos-phorylation is highly dynamic and dependent upon the temporal and spatial balance between kinases and phos-phatases in the cell.
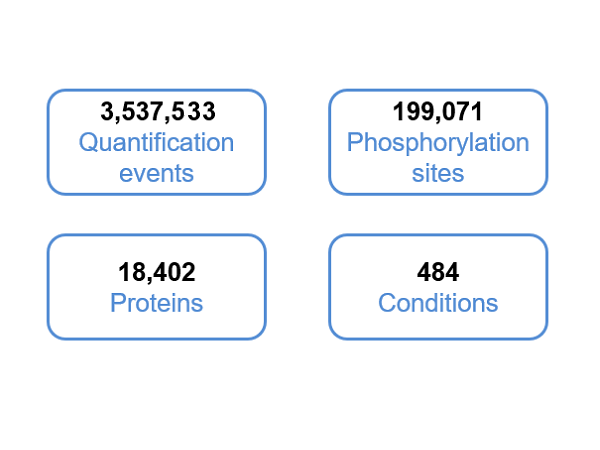
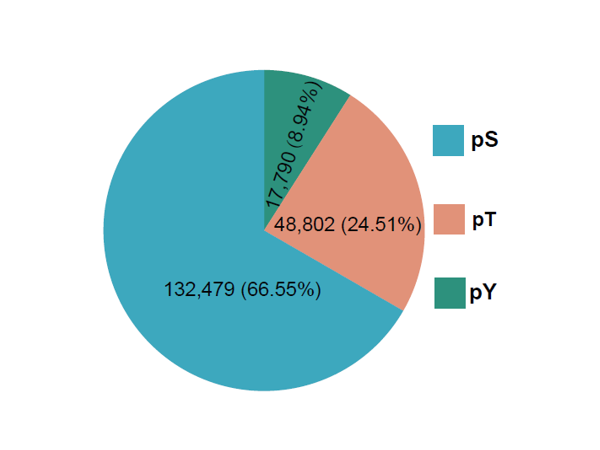
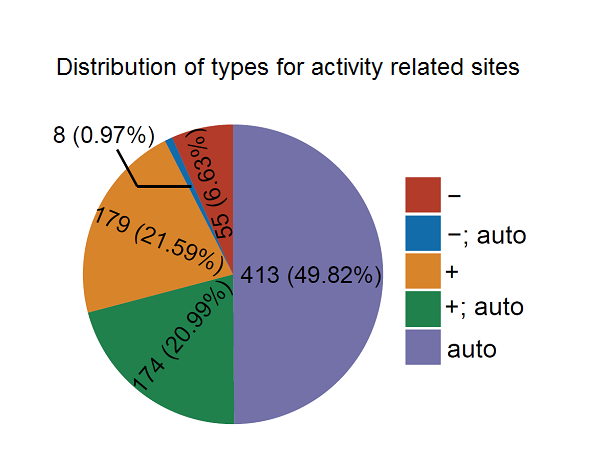
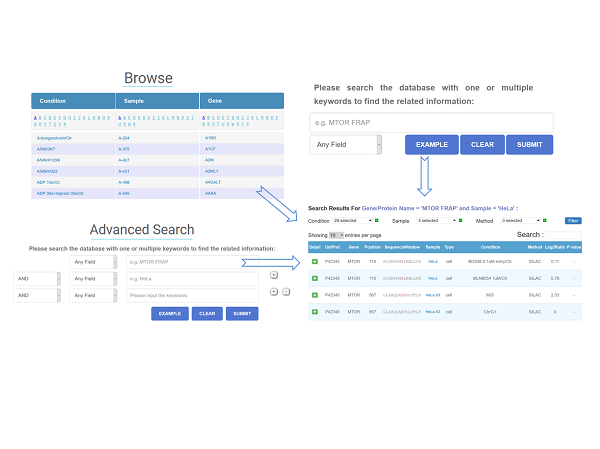
The qPhos database provides the curated quantita-tive phosphoproteome datasets in human tissues and cell lines from published literatures to present the phos-phorylation dynamics under different conditions. In total, 3,537,533 quantification events for 199,071 sites on 18,402 proteins under 484 conditions are collected and integrated in the database.
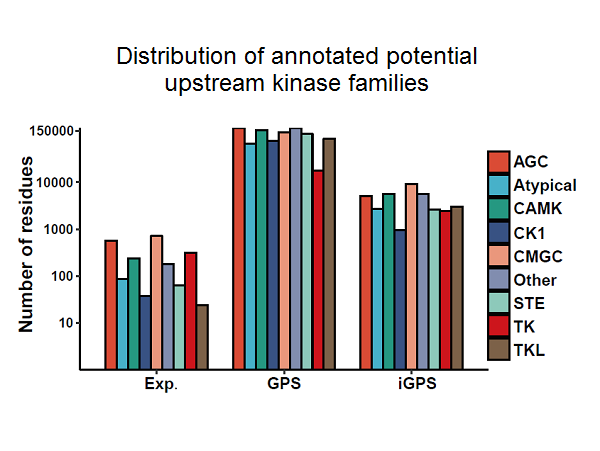
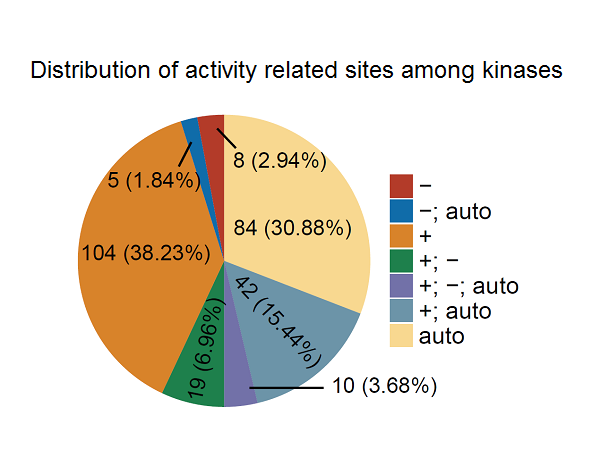
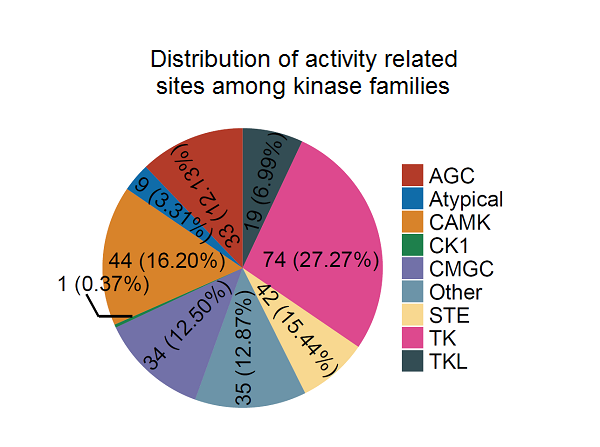
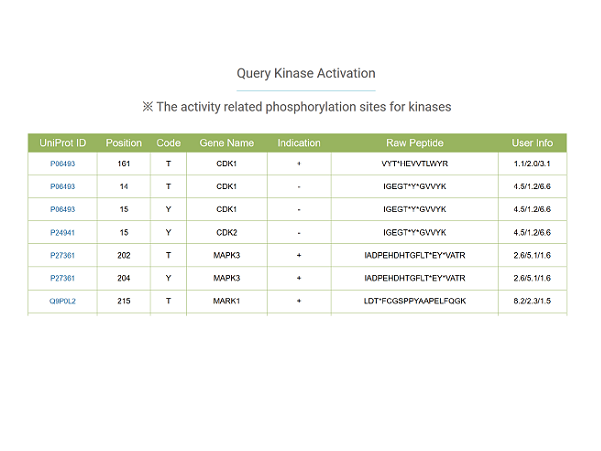
The qKinAct service is developed for dissecting the kinase activity profile from usersubmitted quantitative phosphoproteome data through annotating the kinase act-ivity related phosphorylation sites.
Citation: Yu et al. Nucleic Acids Research, 2019